This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Race Investor Update March 2021
- Race updates its Three Pillar Strategy, detailing intended programs and activities
- The update confirms progress on all Three Pillars, and shares concrete steps towards increasing value for Bisantrene, through its application to broader cancer indications
- Committed programs are funded through calendar 2021
31 March 2021 – Race Oncology Limited (“Race”) is pleased to update our shareholders on progress of our clinical and preclinical programs under the Three Pillars strategy, originally outlined at the Annual General Meeting of shareholders on 30 November 2020. Today’s update confirms committed and planned programs to maximise the significant opportunity offered by Bisantrene as both a targeted precision oncology drug and as a differentiated chemotherapeutic, with the objective of delivering better therapeutic outcomes for patients.
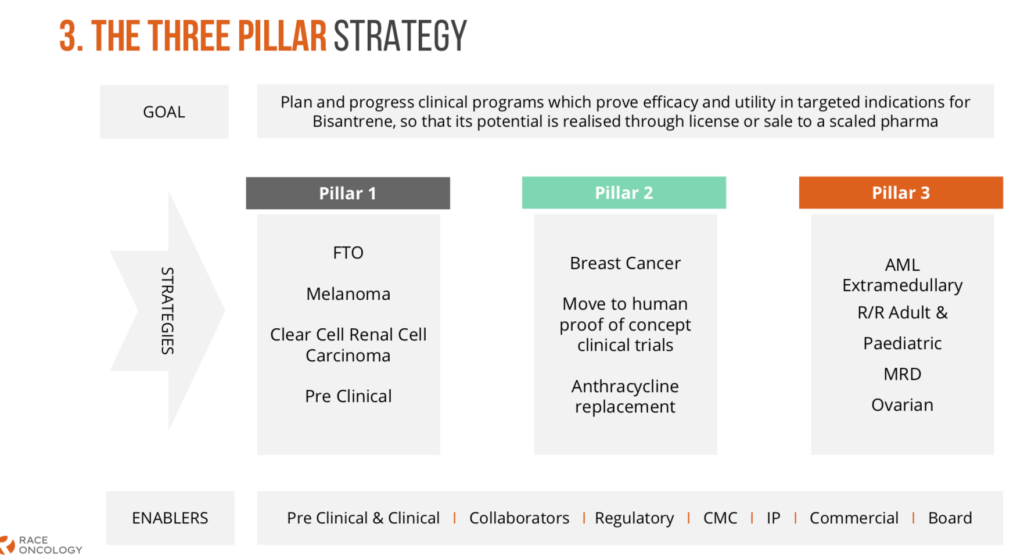
Pillar 1 – FTO (Fat mass and obesity associated protein), a transformative opportunity
At the 2020 AGM Race announced its Pillar 1 program focused on Bisantrene’s ability to act as a potent inhibitor of FTO, a protein understood to play a key central role in the proliferation of many cancers. Two clinically relevant proof-of-concept cancer indications were identified, melanoma and clear cell renal cell carcinoma (ccRCC). Our intention was to undertake preclinical studies in both these indications to support the initiation of FTO-directed Phase I/II clinical trials in CY 2022.
FTO-directed melanoma pre-clinical study
In March 2021 Race announced that renowned melanoma cancer researchers, Professor Xu Dong Zhang and Associate Professor Lei Jin of the University of Newcastle would undertake a collaborative preclinical melanoma research program (ASX announcement: 19 March 2021).
The program’s purpose is to explore the use of Bisantrene as a novel FTO-directed treatment for melanoma using cellular and mouse models to identify drug combinations that improve melanoma treatment, with a focus on treatment resistant cancers. Previous independent published studies have observed that FTO is overproduced in approximately 50% of metastatic melanoma cancers and that the inhibition of FTO can overcome PD-1 immune checkpoint inhibitor resistance in mouse models of melanoma.
This preclinical project will report results over the coming 12 months, which will be shared with the market as received. The results of this study will support a Phase II FTO-directed human trial of Bisantrene in melanoma, currently scheduled to begin in Australia in CY 2022.
FTO-directed clear cell Renal Cell Carcinoma pre-clinical (ccRCC) study
In March 2021 Race announced eminent cancer researcher, Associate Professor Nikki Verrills, who successfully ran Race’s preclinical breast and ovarian cancer programs, would undertake a collaborative preclinical ccRCC-focused research program (ASX announcement: 25 March 2021).
The purpose of this research program is to use cellular models to explore the potential to use Bisantrene as a FTO-directed treatment of ccRCC, a devastating form of kidney cancer. Previous studies have observed that FTO enzyme activity is essential for ccRCC cell survival and the inhibition of FTO can directly kill more than 90% of ccRCC cancers.
The results of this study will support a Phase II FTO-directed human trial of Bisantrene in ccRCC patients, currently scheduled to begin in Australia in CY 2022. This preclinical project will report results over the coming 12 months, which will be shared with the market as received.
Other activities
Race is exploring the initiation of a Phase I dose escalation clinical safety trial of Bisantrene in CY 2021, directed at showing its capacity to inhibit FTO in patients with advanced cancers. Founded upon supportive data, such a trial could accelerate the Phase II proof-of-concept trials planned for CY 2022. Race will update the market on the progress of this proposal once finalised.
Pillar 2 – Breast Cancer and Bisantrene as a differentiated chemotherapeutic
In December 2020 Race tasked the clinical research organisation (CRO), George Clinical, with advisement on an optimal clinical trial design for the Pillar 2 breast cancer program (ASX announcement: 26 November 2020). After extensive consultation with Race’s clinical management team, George Clinical, and key opinion leaders (KOLs) in the breast cancer space, together with data from Race’s recent preclinical breast cancer program (ASX announcements: 24 November 2020 and 9 March 2021), a two-path clinical program has been developed.
Australian Phase II trial in late-stage metastatic breast cancer patients
The first path involves initiation of a Phase II clinical trial of Bisantrene in late-stage metastatic breast cancer patients (who have had three or more lines of prior therapy) in Australia, leveraging Race’s recent preclinical data that demonstrated Bisantrene can kill breast cancer cells resistant to current standard-of-care drugs. These data support the potential clinical utility of Bisantrene in treating late-stage breast cancer patients with treatment resistant tumours.
A Principal Investigator (PI) has been recruited to run this Phase II trial and advanced preparation for this trial has been completed including trial design, synopsis generation, CRO selection, patient inclusion/exclusion criteria, budgeting, and clinical trial site selection.
Final contract signing and initiation of this trial is expected to be completed in Q2 CY 2021 and patient treatment is expected to begin in the second half of CY 2021. Further updates to the market on this trial program will be made over CY 2021.
European Phase IIb trial in anthracycline-naïve metastatic breast cancer patients
Discussions with key opinion leaders in the breast cancer field identified an important clinical need to further demonstrate the historical heart safety of Bisantrene in a modern treatment environment if Bisantrene is to be used as a frontline replacement for the current cardiac muscle-damaging anthracycline chemotherapeutics. Patient exposure to anthracyclines can result in both acute cardiac muscle damage at the time of treatment, as well as a delayed damage response that can take months or even years to manifest1. To provide robust data on cardiac safety it is essential that Bisantrene is trialled in a patient population that has not been previously exposed to anthracycline chemotherapeutics. Such a patient population is not recruitable in Australia. Initial research has identified Eastern Europe as being able to provide anthracycline treatment naïve breast cancer patients.
After detailed discussion with a number international breast cancer KOLs, George Clinical has been tasked by Race with developing and costing a Phase IIb double-blinded clinical trial of Bisantrene verses doxorubicin in geographies where anthracycline naïve patients can be recruited.
George Clinical will provide a detailed, costed proposal to enable gated decision making around the potential to run such a Phase IIb clinical study. This report is expected within three months and Race will update the market once received.
1Kimmick, G., Dent, S., & Klem, I. (2019). Risk of Cardiomyopathy in Breast Cancer: How Can We Attenuate the Risk of Heart Failure from Anthracyclines and Anti-HER2 Therapies? Current Treatment Options in Cardiovascular Medicine, 21(6), 30.
Other Activities
Race is exploring low-cost collaborative preclinical studies focused on understanding the molecular mechanism of action of Bisantrene’s heart safety profile. Advanced discussions are underway with a leading Australian team specialising in anthracycline cardiotoxicity research. Race expects to update the market on the outcome of these discussions in early Q2 CY 2021.
Pillar 3 – Acute Myeloid Leukaemia (AML), an FDA approval pathway
Significant progress has been made on the Pillar 3 program during the quarter. Race has committed in principle support to initiate in 2021 two Phase I/II clinical trials in relapsed/refractory acute myeloid leukaemia (r/r AML) in Australia and Israel. In addition, significant progress has been made in exploring the potential use of Bisantrene in paediatric AML that may enable Race to generate the required data needed for award of a Paediatric Priority Review Voucher (ASX announcement: 18 July 2018). Previous vouchers have been sold on the secondary market for between US$80 and US$200 million.
Phase I/II clinical trial of Bisantrene in combination with Clofarabine and Fludarabine in relapsed or refractory AML (Israel)
Race has reached in principle agreement with Prof Nagler of the Chiam Sheba Medical Center to undertake an open label Phase I/II combination clinical trial of Bisantrene in r/r AML patients.
Prof Nagler led the Phase II single agent open label r/r AML trial of Bisantrene which reported a 40% clinical response rate in a very difficult to treat population of r/r AML patients (ASX announcement: 16 June 2020).
This Phase I/II trial will enrol 29 patients and involve the use of Bisantrene in combination with the nucleoside analogues, clofarabine and fludarabine. Unpublished preclinical data from Professors Andersson and Valdez of the MD Anderson Cancer Clinic in Houston, Texas has identified strong synergistic killing of AML cells using this three-drug combination.
Human ethics approval for this trial has been granted and the first patient is expected to be treated in Q2 CY 2021. The trial is scheduled to run for up to 36 months, but Race will release progress updates on patient responses as they are received.
Trial contract details are currently being finalised and Race expects to sign in early Q2 CY 2021.
Phase I/II Clinical Trial of Bisantrene in Extramedullary AML (Australia)
Race has reached in principle agreement with Associate Professor Anoop K Enjeti, Director of Haematology at the Calvary Mater Newcastle and John Hunter Hospitals, to undertake an open label Phase I/II clinical trial of Bisantrene in patients with the extramedullary form of AML. Extramedullary AML occurs when the leukaemia spreads from the bone marrow and forms solid tumours in tissues such as the skin, breast, kidney, brain, or other organs. A 2020 prospective positron imaging trial identified that up to 22% of AML patients have the extramedullary form.
Dr Enjeti is a highly experienced clinical haematologist having designed and led more than 25 clinical trials. Dr Enjeti is the co-chair of the MDS/AML working party for the Australasian Lymphoma and Leukemia Group (ALLG) for Cooperative Clinical trials. He has published more than 45 papers in the haematology field and attracted more than $3.5 million in grant funding.
This two-armed Phase I/II trial will involve the use of Bisantrene as a high dose single agent treatment of extramedullary AML patients able to endure high intensity chemotherapy, or alternatively as a low dose Bisantrene treatment in combination with a hypomethylating agent in the less fit patients. The trial will recruit 40 patients at 10 clinical sites across Australia and New Zealand.
The trial is expected to received human ethics approval in Q3 CY 2021 and treat the first patient in late 2021. The trial is expected to run for 18 months. Results of this trial will support parallel pivotal Phase II/III clinical trials in the USA and EU with the aim of achieving rapid FDA/EMA label approval of Bisantrene for use in r/r AML. Furthermore, this trial may be expandable into a paediatric extramedullary AML population and enable Race to receive a US Paediatric Priority Review Voucher.
Phase I/II clinical trial of Bisantrene in combination with Fludarabine and Cytarabine for children with r/r AML (USA)
Race is in advanced evaluation on the prospect of initiating a pivotal Phase I/II trial of Bisantrene in combination with fludarabine and cytarabine in children with first or second relapse of AML. In principle support has been received from the USA Children’s Oncology Group (COG) and a proposed trial design has been provided to Race by COG for evaluation. The trial is expected to run for 36 months and requires FDA IND approval before it can be initiated.
While this trial may enable Race to meet the conditions required to be granted a Paediatric Priority Review Voucher, additional research and communication with the FDA is required to determine if the trial can be completed before the September 2026 sunset of the Rare Paediatric Disease Voucher program. Race will update the market as to the outcome of this research when complete.
Preclinical Extramedullary AML Study
In March 2021 Race announced Associate Professor Nikki Verrills, would undertake a collaborative preclinical extramedullary focused research program (ASX announcement: 30 March 2021).
The aim of this project is to support the clinical use of Bisantrene as a novel treatment for extramedullary AML, a difficult to treat form of AML, using an extramedullary mouse model developed by A/Prof Verrills’ team.
The results of this study will provide support for a pivotal (Phase II/III) trial of Bisantrene in extramedullary AML patients with the aim of providing a rapid path to FDA approval for Bisantrene as an orphan drug under the 505(b)(2) track.
This project is to start immediately with the results expected to be reported over the coming 12 months.
“Following on from the November 2020 AGM, we have evaluated how to best maximise the exceptional asset that Bisantrene represents. The 2021 program capitalises on Bisantrene’s significant FTO opportunity while also pursuing its differentiated chemotherapy profile. We propose that the strategy outlined today minimises risk while maximising commercial and therapeutic upside, particularly with respect to the potential we see in FTO. The 2021 committed program is fully funded and will generate actionable data by year-end, so supporting an expanded 2022 clinical program thereafter.”
Chief Executive Officer, Mr Phillip Lynch and Chief Scientific Officer, Dr Daniel Tillett
Shareholders can expect continued updates to results as they read out, and in later 2021 specific communication on expanded FTO-focused clinical programs.


