This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Zantrene kills melanoma cancer cells that overproduce FTO
- Zantrene at low concentrations kills high FTO producing melanoma cancer cells
- Sensitivity to Zantrene correlates with FTO levels, where high FTO producing cells show up to 60x greater sensitivity than low FTO producing cells
- Results are highly supportive of future clinical trials in melanoma using Zantrene in combination with standard of care treatments.
Race Oncology Limited (“Race”) is pleased to share interim results from our collaborative preclinical melanoma research program with the University of Newcastle (ASX Announcement: 19 Mar 2021). Eminent melanoma researchers, Professor Xu Dong Zhang and Associate Professor Lei Jin, are leading the project.
This program is exploring the use of Zantrene® (bisantrene dihydrochloride) as a novel potential treatment for melanoma using cellular and mouse models. The aim is to identify drug combinations and melanoma subtypes that show improved treatment responses, with a focus on treatment-resistant melanomas.
These interim results showed Zantrene® to be highly effective at killing a diverse range of high FTO producing melanoma cell subtypes. Data from the expression of the Fat Mass and Obesity-associated protein (FTO) showed an association between FTO expression level and sensitivity to Zantrene.
Zantrene has been identified as a potent targeted inhibitor of the Fat Mass and Obesity associated protein (FTO)1. Previous studies have observed that FTO is over-produced in approximately 50% of metastatic melanomas2 and that inhibition of FTO can overcome PD-1 immune checkpoint resistance in mouse melanoma models2,3. PD-1 immune checkpoint inhibitors have emerged as a front-line treatment for many types of cancer, including melanoma. While there have been major advances in melanoma treatments in recent decades, the five-year survival rate for advanced melanoma remains low4.
“These interim results are highly encouraging and support our clinical plans for Zantrene, with the correlation between FTO overexpression and sensitivity to Zantrene suggesting a strong anti-FTO therapeutic opportunity. The high sensitivity of many of the melanoma cell lines to Zantrene as a single agent at concentrations well below chemotherapeutic doses is unexpected and may offer new treatment options for melanoma patients.”
Race CSO Dr Daniel Tillett
“While challenged by COVID 19 related shutdowns we appreciate the encouraging and continued work from the team at the University of Newcastle. Zantrene continues to positively surprise us – we are very pleased with these early results. Melanoma remains a difficult cancer to treat, and one that’s of particular relevance to the Australian community, so as we continue with this work, we look forward to learning more about our potential to offer new treatment options to patients.”
Race CEO & MD Phillip Lynch
Study Background
Melanoma is unresponsive to existing anthracyclines, yet Zantrene showed significant historical in vitro activity against fresh human melanoma samples taken from patients in human tumor cloning assays5,6. In a subsequent Phase I trial of Zantrene administered weekly, a patient with metastatic melanoma achieved a complete response lasting 6 months7. This weekly dosing schedule would likely have resulted in sustained inhibition of FTO due to the long time Zantrene remains in the human body.
Despite these early successes, four subsequent Phase 2 studies of Zantrene in 100 melanoma patients used much longer dosing intervals of once every three or 4 weeks and did not achieve the same levels of clinical response, possibly due to limited, transient inhibition of FTO8-11. Seventeen patients (1/16, 2/16, 0/17, 14/51) achieved disease stabilization, but no further complete responses were observed.
In light of the recent discovery that Zantrene is a potent inhibitor of the m6A RNA demethylase FTO1 and that FTO is frequently overexpressed in metastatic melanoma2, Race sought to explore the use of Zantrene for treating melanoma, both as a single agent and in combination with other standard of care drugs. This research will also help inform the dose regimen to be explored in future clinical trials.
Study Highlights
1. Zantrene® is highly effective in killing melanoma cells at sub-chemotherapeutic levels
Zantrene proved to be highly effective at killing melanoma cell lines, with 60% (15 of 25) displaying IC50 values below 100 nM concentrations (Table 1). This was seen with cell lines derived from both primary and metastatic melanoma patients. Interestingly, six of the 25 cell lines showed extreme sensitivity to Zantrene (IC50 values under 40 nM), suggesting that Zantrene may provide an effective single agent treatment for some patients.
The untransformed melanocyte cell line (normal) was highly resistant to cell killing by Zantrene, as were some of the melanoma cell lines (Table 1). The molecular mechanisms underlying this resistance to Zantrene remain to be determined.
| Cell Line | IC50 (nM) |
| HEMm-MP | 1403 |
| MEL-BP | 1003 |
| SK-MEL-110 | 1002 |
| SK-Mel-28 | 515 |
| Mel-JD | 335 |
| MM426 | 312 |
| Mel-RM | 178 |
| Mel-RMu | 167 |
| Mel-CV | 115 |
| Mel-FH | 102 |
| MM200 | 96 |
| A375 | 87 |
| MM170-5 | 85 |
| MM283 | 79 |
| ME1007 | 78 |
| SK-MEL-37 | 75 |
| IgR3 | 72 |
| SK-MEL-13 | 67 |
| ME4405 | 53 |
| MEL-BE | 39 |
| MV3 | 39 |
| MEL-EH | 37 |
| MEL-JR | 30 |
| MEL-KD | 28 |
| MM962 | 23 |
Table 1. IC50 values for Zantrene®
2. Sensitivity to Zantrene® is independent of BRAF and NRAS mutational status
Zantrene sensitivity did not show any correlation to either BRAF or NRAS mutational status, with individual mutant and wild type cell lines displaying a wide range of IC50 values. This result suggests that Zantrene® may show utility in patients resistant to BRAF and NRAS inhibitors.
| Cell Line | IC50 (nM) | BRAF | NRAS |
| HEMn-MP | 1403 | Wild | Wild |
| SK-Mel-28 | 515 | Mutant | Wild |
| Mel-JD | 335 | Wild | Mutant |
| Mel-RM | 178 | Wild | Mutant |
| Mel-Rmu | 167 | Mutant | Wild |
| Mel-CV | 115 | Mutant | Wild |
| Mel-FH | 102 | Wild | Wild |
| ME1007 | 78 | Wild | Wild |
| MM200 | 96 | Mutant | Wild |
| IgR3 | 72 | Mutant | Wild |
| ME4405 | 53 | Wild | Mutant |
3. Sensitivity to Zantrene® correlates with FTO expression levels
Zantrene sensitivity was correlated with FTO protein overexpression levels (Figure 1 and Table 3).
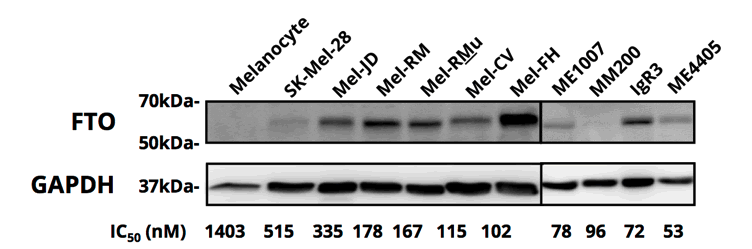
The eight most Zantrene-resistant cell lines (i.e. those with an IC50 values greater than 100 nM) had a median FTO expression level 1.4 fold higher that of the untransformed (normal) melanocyte cell line, HEMn-MP.
In contrast, the 15 most sensitive melanoma cell lines had a median increase in FTO protein levels of 2.5 fold. The five most sensitive cell lines (i.e. those with IC50 values below 40 nM) had an average increase in FTO protein levels 3.8 fold higher (Table 3).
| Cell Line | FTO Level |
| HEMm-MP | 1.0 |
| Mel-BP | 2.2 |
| SK-MEL-110 | 5.0 |
| SK-Mel-28 | 1.0 |
| Mel-JD | 1.3 |
| MM426 | 2.8 |
| Mel-RM | 1.6 |
| Mel-RMu | 1.4 |
| Mel-CV | 1.4 |
| Mel-FH | 2.6 |
| MM200 | 1.1 |
| 70W | 1.9 |
| MM170-5 | 3.5 |
| MM283 | 2.4 |
| ME1007 | 1.3 |
| SK-MEL-37 | 2.4 |
| IGR3 | 2.7 |
| SK-MEL-13 | 4.4 |
| ME4405 | 2.0 |
| Mel-BE | 1.7 |
| MV3 | 5.8 |
| Mel-EH | 2.2 |
| Mel-JR | 3.6 |
| Mel-KD | 2.5 |
| MM962 | 5.0 |
The SK-MEL-10 was an exception to the trend of high FTO overexpression being associated with high sensitivity to Zantrene. This cell line had an IC50 value over 1000 nM while displaying an FTO expression level 5 fold that of HEMn-MP. The reason for this resistance is unknown, but may be linked to high expression of drug efflux pumps like MDR1 that are known to reduce the intracellular concentration of Zantrene.
Conclusions
- Zantrene showed unexpectedly effective killing of melanoma cell lines at concentrations well below 100 nM (sub-chemotherapeutic), with a number of cell lines displaying very high sensitivity (less than 40 nM)
- Zantrene proved effective at killing melanoma cell lines with BRAF or NRAS mutations
- Sensitivity to Zantrene was correlated with overproduction of the FTO protein, supporting Race’s clinical plans for using Zantrene in combination with standard of care drugs for the treatment of melanoma patients.
References
- Su, R., Dong, L., Li, Y., Gao, M., Han, L., Wunderlich, M., et al. (2020). Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell, 38(1), 79–96.e11.
- Yang, S., Wei, J., Cui, Y.-H., Park, G., Shah, P., Deng, Y., et al. (2019). m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nature Communications, 10(1), 1131–14.
- Li, N., Kang, Y., Wang, L., Huff, S., Tang, R., Hui, H., et al. (2020). ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proceedings of the National Academy of Sciences, 117, 20159–20170.
- www.cancer.net/cancer-types/melanoma/statistics
- Hoff, D. D. V., Coltman, C. A. & Forseth, B. (1981). Activity of 9–10 anthracene dicarboxaldehyde-bis[(4,5-dihydro-1-H-imidazol-2-yl)hydrazone] dihydrochloride (CL216,942) in a human tumor cloning system. Cancer Chemoth Pharm 6, 141–144.
- Salmon SE, Meyskens Jr FL, Alberts DS, Soehnlen B, Young L. (1981). New drugs in ovarian cancer and malignant melanoma: in vitro phase II screening with the human tumor clonogenic cell assay. Cancer Treat Rep 65, 1-12.
- Alberts, D. S., Mackel, C., Pocelinko, R. & Salmon, S. E. (1982). Phase I clinical investigation of 9,10-anthracenedicarboxaldehyde-bis[(4,5-dihydro-1H-imidazol-2-yl)hydrazone] dihydrochloride with correlative in vitro human tumor clonogenic assay. Cancer Res 42, 1170–5.
- Mackel, C., Meyskens, F. L. & Alberts, D. S. (1986). Phase II trial of bisantrene in patients with metastatic melanoma. Cancer Treat Rep 70, 1037–8.
- Stiff, P. J. et al. (1991) Phase II trial of bisantrene for metastatic melanoma: An illinois cancer council study. Med Pediatr Oncol 19, 126–128.
- Coates, A. S., Bishop, J., Mann, G. J. & Raghavan, D. (1986). Chemotherapy in metastatic melanoma: Phase II studies of amsacrine, mitoxantrone and bisantrene. European J Cancer Clin Oncol 22, 97–100.
- Alberts, D. S. et al. (1987). Phase II evaluation of bisantrene hydrochloride in refractory malignant melanoma. A Southwest Oncology Group Study. Investigational New Drugs 5, 289–292.